Biomolecules Notes Class 11 & Class 12 with Question Answers

Do you want Biomolecules Notes PDF? If so, you are exactly in the right place. Most of the people think this is a hard chapter, in fact it is not.
Biomolecules PDF download link is provided at the end of this article. You can choose to save this free pdf for offline use or also can read this PDF online here. This pdf contains both notes and questions answers.
Biomolecules Notes PDF
In biology and bio-chemisrty, biomolecules mean small particles that can be either charged organic molecules or compounds. These are made and found in living things, like proteins, carbs, DNA, and so on.
Biomolecules come in a wide range of shapes and sizes, and they do a lot of different things. A biomolecule is one of the many chemicals that cells and living things make.
The major types of Biomolecules includes:
- Carbohydrates
- Lipids
- Nucleic acids
- Proteins
- Amino Acids
What are Organic Compounds?
Carbon, hydrogen, oxygen, and nitrogen combine to create organic molecules. Organic compounds is mainly categorised into macro-molecules and micro-molecules.
Micro-molecules are molecules with a low molecular weight and a simple structure. fatty acids and monosaccharides.
Macromolecules are molecules with a large molecular weight and complicated structure. E.g. Polysaccharides, DNA etc.
1. Carbohydrates
Carbohydrates are made up of carbon, hydrogen, and oxygen. Where hydrogen and oxygen are 2:1 as in water. There are major four types of carbohydrates:
Monosaccharides: the simplest carbohydrate type. Glucose, fructose, and other sugars are water soluble and pleasant in flavour.
Disaccharides: Disaccharides are produced by two monosaccharide molecules. Eg: Maltose, sucrose, lactose. They are water soluble and taste sweet.
Oligosaccharidesl: Starch is the example of this carbohydrate. Plants retain starch as a food source.
Polysaccharides: Polysaccharides are made up of millions of monosaccharides.
2. Proteins
Proteins are made of carbon, oxygen, nitrogen, hydrogen and sulphur. They also have phosphorus, iron, calcium, iodine, and copper residues in them, as well as leftovers from other things like amino acids. Protein is about 15% of a cell's "living" protoplasm.
It's because the chemical substances that make up protein molecules, called amino acids, can be different. Proteins are biomolecules that can have different structures. There are new bonds made when there are many new peptides in a string together.
Proteins are classified by how they look, how they work, and how they are made. This word is also known as a "peptide," but it's not the same as a "polypeptide," "tripeptide," or "dipeptide." Proteins can be simple or conjugated based on how many parts they have.
Proteins are classified as acidic or basic based on their molecular composition. Scleroprotein Fibrous Protein and Globular Protein are two types of protein.
3. Nucleic Acids - RNA/DNA
Nucleic acids are the genetic substance in cells that transmits genetic information from parents to offspring. Translation and transcription are two major functions of nucleic acid. There are two kinds of nucleic acids: DNA and RNA (RNA).
The pentose sugar's nitrogen base distinguishes the nucleotide. DNA has four nitrogenous bases: adenine, guanine, cytosine, and thymine. The nucleotide is made up of a nitrogenous base, pentose sugar, and phosphate. 3' and 5' phosphodiester bonds connect the nucleotides.
The DNA structure resembles a twisted ladder. In RNA, thymine is uracil. Double-helical structure produced by hydrogen bonding between bases of two antiparallel polynucleotide strands.
4. Lipids
Lipids are not polymeric like carbohydrates, proteins, and nucleic acids. Lipids are chemical compounds that are linked to fatty acids and used by living cells. These fatty acids are essential for cellular structure and energy. The fats, waxes, sterols, fat-soluble vitamins, and phospholipids are examples.
5. Amino Acids
There are 20 amino acids in nature. Essential and non-essential amino acids are classified by the body's ability to synthesise them. It has an acidic carboxyl (-COOH) and a basic amino (-NH2) group. They have a carbon, hydrogen, and oxygen group. Proteins are made up of amino acids.
Difference between Non essential protein and essential protein:
| Essential Proteins | Non-Essential proteins |
| 1. Can not be synthesized in our body. 2. These amino acid are not already available so, need food supply. 3.Examples: leucine, threonine, valine | 1. Can be synthesized in our body. 2. These amino acids are already found in the body in enough amount. 3. Examples: aspartic acids, glutamic acid, tyrosine, glycine, ornithine, arginine, histidine, taurine, serine, alanine. |
DNA's Watson-Crick Model (1953)
The bases are A, C, G, and T; A and T are always paired, as is C and G. According to this model, DNA is a double-stranded helical molecule. It has two sugar-phosphate backbones on the exterior and two nitrogenous bases on the interior. These pairing principles indicated that either strand might create a new duplicate of the whole molecule, and that the aperiodic arrangement of bases could give a "genetic code."
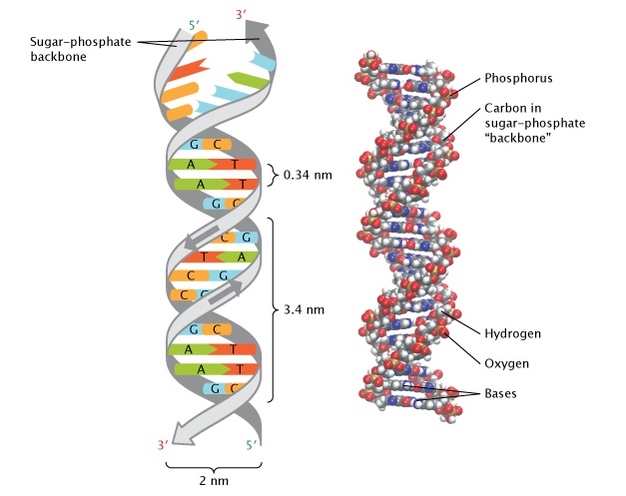 |
| Image Credit - Nature.com |
With Maurice Wilkins (1916-2004), Watson and Crick shared the Nobel Prize in 1962 for their discovery. Rosalind Franklin (1920 - 1958), also working in the same lab, had previously discovered a helical structure. On to molecular and neurobiological research. Watson directed the Human Genome Project in the 1990s.
To know which type of questions might be asked from this chapter in biology exam, you should see Class 11 Biology Model Question Paper.
Biomolecules Questions and Answers:
1. Define Macromolecules.
Ans. Macromolecules are polymerised biomolecules that are formed from many molecules with a high molecular weight. Simply, macromolecules are huge biomolecules. Their molten weight ranges from 18 to 800 daltons (Da). Though macromolecules are bigger than ordinary molecules, they are invisible to the human eye. These polymeric complex macromolecules are found colloidally in the intercellular fluid. Proteins, nucleic acids, polysaccharides, etc. DNA, RNA, Nylon, Polyester, Keratin in hair, waxes, oils, steroids, grease, hormones, etc.
2. Can Protein Be Used Therapeutically? What Other Uses Does Protein Have? List.
Ans. Proteins may be utilised therapeutically. Proteins have been synthesised in the lab for various medicinal purposes. Therapeutic proteins include:
- Antigens
- Diastase
- Streptokinase
- Renin
- Vasopressin
- Insulin,
- Using antibodies
- Enzymes,
- Proteases,
- BMPs,
- Protein scaffolds.
In addition, they have solubility, cell interactions, tertiary structural stability, immunogenicity, and pharmacokinetics of therapeutic proteins having carbohydrate profiles. So they're vital in treating severe illnesses like cancer and HIV. Proteins are also used in cosmetics, textiles, biological buffers, and research. Therapeutic proteins have revolutionised healthcare.
3. Give some examples of how lipids may be found in various forms.
Lipids, or fats, are composed of simple fatty acids. They don't mix with water, existing as fatty acids. Fatty acids have a carboxyl group and an R-group, which can be methyl/ethyl groups or a -CH2 chain.
For example, Arachidonic acid has 20 carbon atoms, while palmitic acid has 16. Unsaturated fatty acids are more common. Glycerol, also known as trihydroxy propane, is another type of lipid. Fatty acids and glycerol form diglycerides, triglycerides, and monoglycerides (oils or fats based on melting point), with diglycerides being the most common.
Phospholipids, which contain phosphorus and can be found in cell membranes and throughout the body, and phosphorylated organic compounds are other types of lipids, with lecithin being an example of a phospholipid.
4. Can you tell about the primary roles of carbohydrates?
Carbohydrates perform the following primary functions:
- The control of blood glucose levels.
- They are the world's most important source of energy. As a result, it is engaged in the breakdown of starch into glucose and the degradation of proteins into energy for metabolism.
- It plays a role in fat metabolism and helps to keep the body out of ketosis.
- Fiber, carbohydrates, and sugars are all essential components of food, serving as the building blocks of the meal.
- Make it possible for the body and neurological system to get energy and nourishment.
5. In catalyzed reactions, the creation of the enzyme-substrate complex is the initial step in the process. Explain the remaining stages leading up to the creation of the final product.
The activity of enzymes is described in the following manner:
- The substrate attaches to the active site of the enzyme first, and then the enzyme binds to the substrate that fits into the active site
- As a result of this interaction, the enzyme changes its shape, allowing it to fit more securely around its substrate.
- The active site of the enzyme breaks the chemical bonds of the substrate in the area of the substrate, resulting in the creation of a new enzyme-product complex in the neighborhood of the substrate.
- The enzyme catalyzes the release of the products of the process. The free enzyme is now ready to attach to another molecule of the substrate of the same type and proceed to the next catalytic cycle of the reaction.
6. Using the Watson-Crick model, describe the secondary structure displayed by nucleic acids in detail.
Watson-Crick model essentially says that DNA exists as a double helix structure, with the two strands of polynucleotides running in the opposite direction of one another.
According to this model, nucleic acids have a broad range of secondary structures in addition to primary structures.
The nitrogen bases are suggested nearly perpendicular to the backbone, facing inwards, and create the sugar-phosphate-sugar chain, which serves as the structure's backbone. The bases A and G on one strand form base pairs with the bases T and C on the other strand.
When connecting A and T, there are two hydrogen bonds, and when connecting G and C, there are three hydrogen bonds, each of which looks as an inverted helical staircase. There are 10 base pairs in one full round of the helical strand.
Hope this article has helped you. You can also download Biomolecules Notes PDF following the instructions given in this article.
In previous article, we have provided origin and evolution notes pdf.